The SciTech Drug Delivery Platform (SDP)
- Designed to safely deliver drugs.
- Works by formulating drugs into very tiny spheres known as nanovesicles.
- Nanovesicles “trick” cells into letting the drug inside.
- Can be designed and engineered with other drugs providing more effective and patentable drugs.
- May have the ability to be used as an adjunct to other therapies.
- Cost-effective, scalable and biocompatible.
- Potentially applicable to other modes of administration such as topical and oral.
Please visit www.SciTechDevelopment.com for more detailed science information.
ST-001: A Breakthrough New Drug
About ST-001 nanoFenretinide
- Our patented lead drug, ST-001 nanoFenretinide uses our Delivery Platform in combination with fenretinide, a drug with a well-documented safety profile.
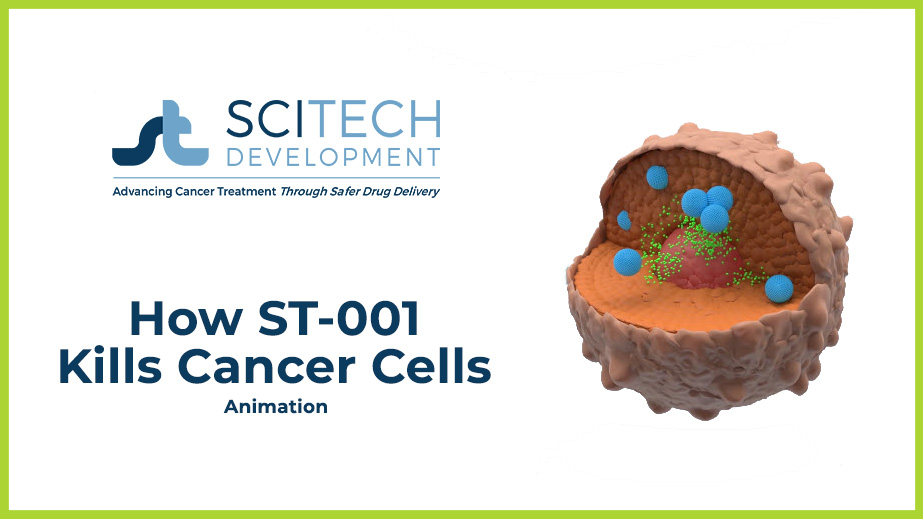
- ST-001 is administered to the patient intravenously (IV).
- Once in the bloodstream, ST-001 nanovesicles bind to the cancer surface and penetrate the cell.
- Once inside the cell, high concentrations of fenretinide are released which disrupts cellular DNA, causing the cancer cell to die.

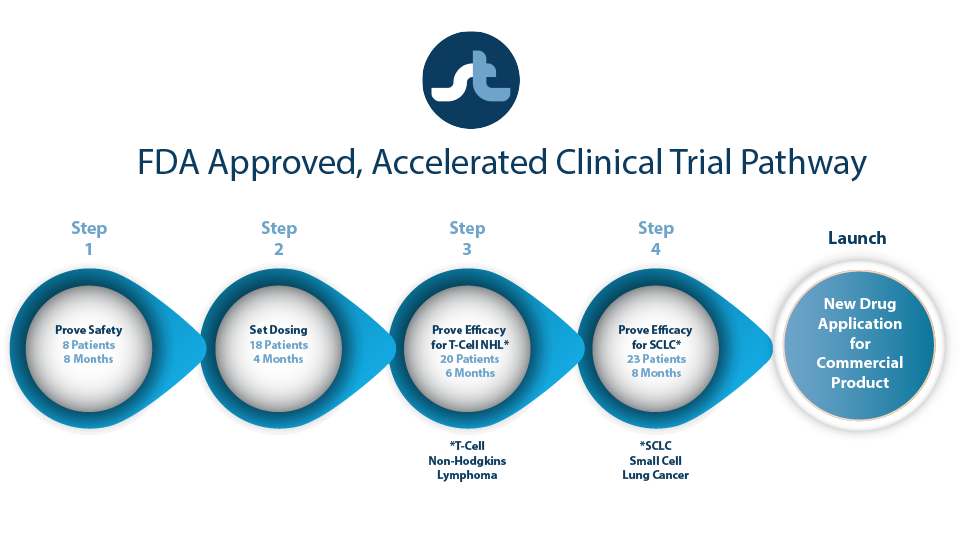
ST-001 is Clinical Trial Ready
Our accelerated, Phase 1 clinical trial utilizes ST-001 nanoFenretinide for the treatment of T-cell lymphomas.
(ClinicalTrials.gov Identifier: NCT04234048).
Our goal is to confirm that:
- ST-001 nanoFenretinide new formulation is safe.
- ST-001 nanovesicle platform can transport fenretinide into the cancer cell at the right dose and without toxic side effects.
- ST-001 provides beneficial patient outcomes.
Fenretinide has also shown promise in the treatment of cervical, colorectal, head & neck, ovarian, pancreatic, prostate, and pediatric cancers.
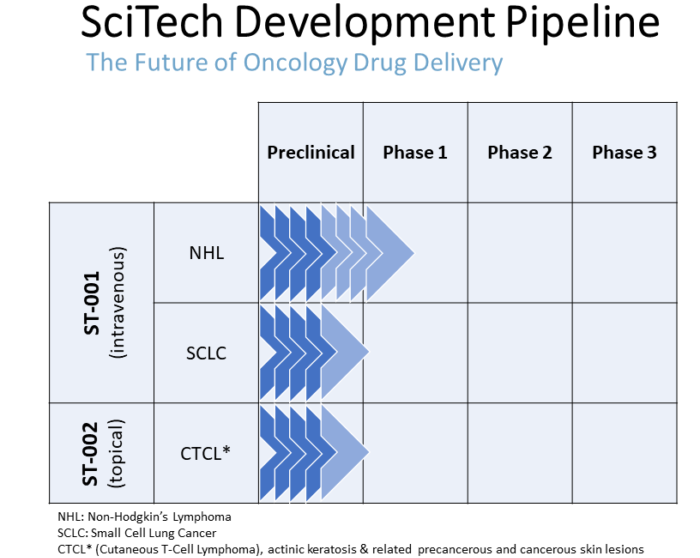
Our Pipeline
SDP holds the promise of enhancing the therapeutic performance of other existing and future cancer and non-cancer drugs that have solubility and bioavailability challenges similar to those of fenretinide.
In addition to our initially targeted indications, we plan to pursue ST-001 nanoFenretinide for the treatment of:
- Small cell lung cancer (SCLC)
- Metastatic breast cancer (MBC)
- Neuroblastoma (pediatric cancer)
- T-cell acute lymphoblastic leukemia (T-ALL)
For more detailed information on our science and our company, please visit www.SciTechDevelopment.com.


