Cancer is a Growing Worldwide Problem
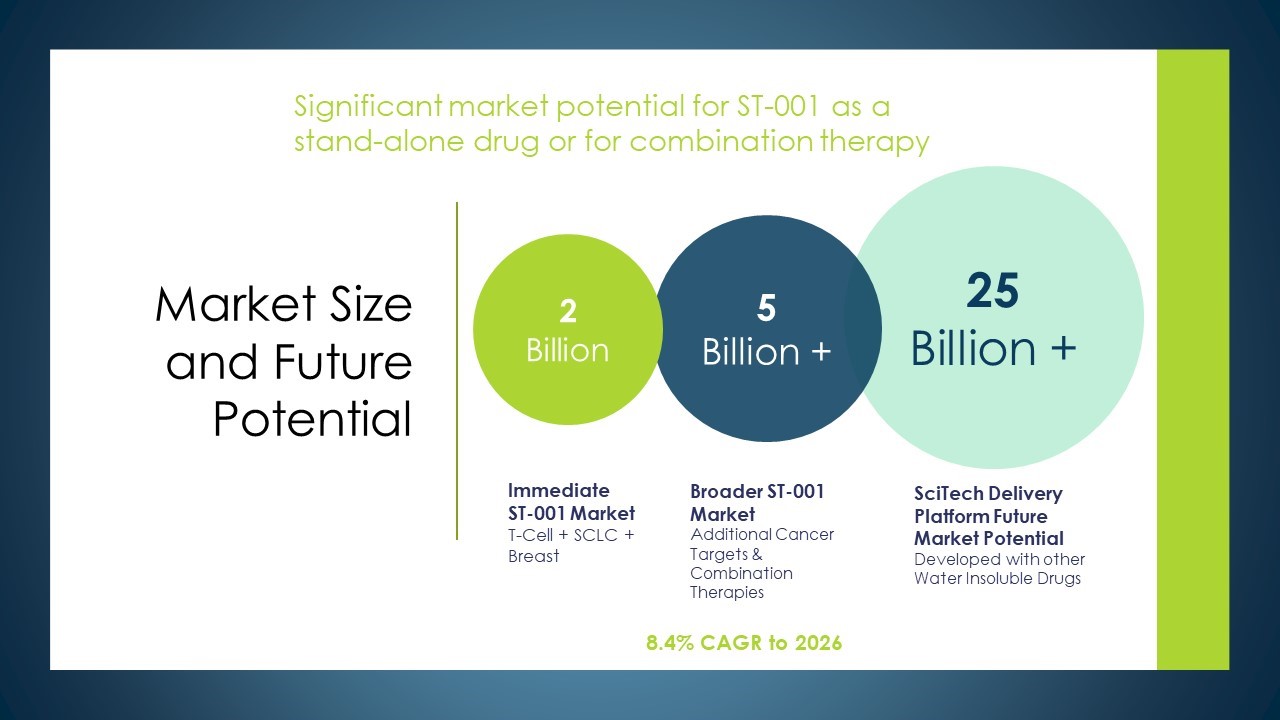
- ST-001 nanoFenretinide, our first, patented drug candidate, represents a vast $2 Billion market potential.
- ST-001 is based upon the safe drug fenretinide which is broadly applicable to several cancer types.
- ST-001 will be an affordable treatment option.
- ST-001 has a clear regulatory path with an accelerated clinical trial plan.
- ST-001’s product development and manufacturing scale-up has been successfully completed.
- We have the support of powerful institutions such as the National Cancer Institute (NCI) who is underwriting the supply of fenretinide for our clinical trials.
- SciTech has several exit options and expansion strategies.


How We’re Different
- Quick Time to Market
- Accelerated Phase 1 trial to confirm safety and demonstrate efficacy
- Low cost of drug development
- Low cost of outpatient administration
- ST-001 is anticipated to have high efficacy and low toxicity (= high therapeutic index)
- Multiple mechanisms of action to target a broad spectrum of cancers with no genetic markers required for use
Our Strategy
- Our accelerated trial plan for ST-001 provides a rapid path to commercialization and revenue generation.
- SciTech will continue to identify and develop additional drug candidates and administration routes to add to our product portfolio.
- We seek companies that want to expand their existing drug portfolio in oncology, target new diseases, or develop combination drug therapies.
- Potential exit strategies include licensing, partnerships, or a targeted acquisition.

Recent Milestones Achieved
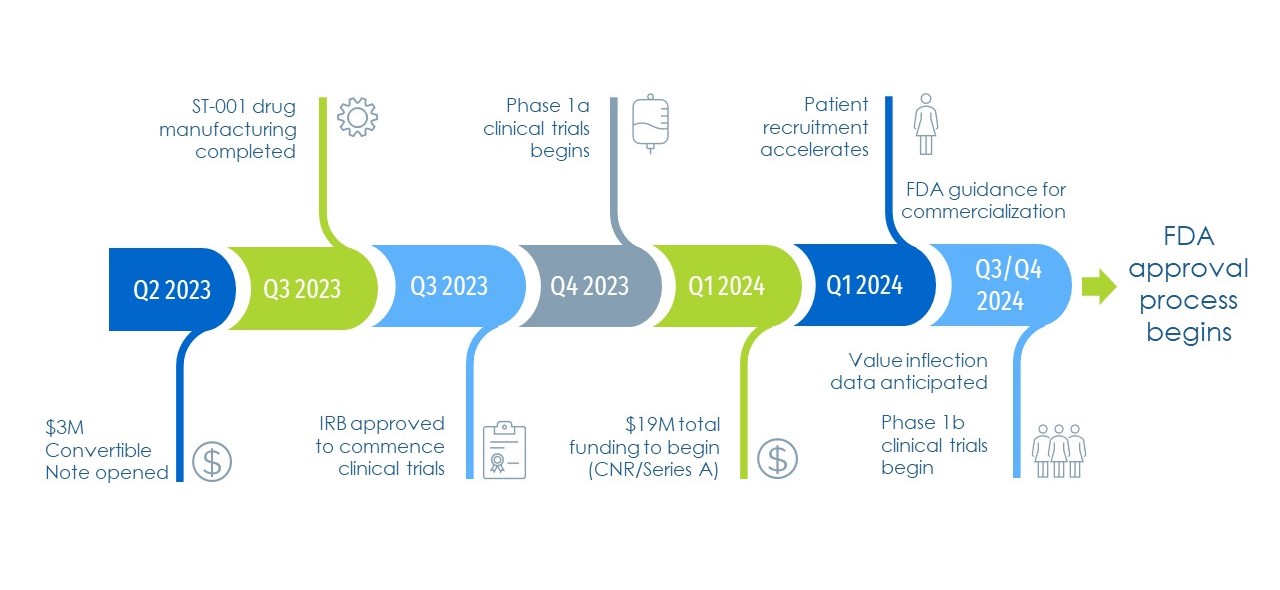
ST-001 commercialization estimated 24 months or less after start of Phase 1a Trial
SciTech has made great strides over the last several years in our quest to bring ST-001 to market.
- U.S. Small Business Innovation Research (SBIR) grants from the National Cancer Institute.
- Investigational New Drug (IND) approval from the FDA, required to start clinical trials.
- FDA guided clinical trial plan: Accelerated time to market and revenue generation.
- FDA Orphan Drug Designation: Providing SciTech with exclusive marketing and development rights, federal tax credits and FDA fee reductions.
- Multiple Institutional Review Board (IRB) approvals for initial clinical trials.
The Market Opportunity
Questions about this investment opportunity?
Please contact David Schaffer for additional information.
InvestorRelations@SciTechSDP.com

